Implementiamo infrastrutture basate su cloud per migliorare la scalabilità, la produttività e la sicurezza delle aziende.
GRATUITO
I nostri workshop online sono gratuiti!
Nell’era digitale, le aziende affrontano costantemente nuove sfide e opportunità. La “Ruota della Trasformazione” di ICAREX é un approccio innovativo per guidare le aziende attraverso un percorso strutturato e circolare che facilita la trasformazione da ogni prospettiva.
ICAREX è un Venture Studio che fa dell'esperienza pratica e della rapidità i propri valori fondamentali. Siamo imprenditori prima che consulenti: ogni nostra soluzione è il frutto diretto di sfide reali affrontate sul campo, garantendo autenticità ed efficacia nel vostro contesto di business. La nostra rete globale di 200+ professionisti consente la creazione di squadre ad hoc in sole 24 ore, fornendo competenze tecnologiche all'avanguardia immediatamente applicabili al vostro business.
Implementiamo infrastrutture basate su cloud per migliorare la scalabilità, la produttività e la sicurezza delle aziende.
Sfruttiamo la bioinformatica per estrarre dati illuminanti dalle informazioni biologiche, guidando l’innovazione in molteplici settori.
Guidiamo le aziende nella creazione di esperienze coinvolgenti nel metaverso, coinvolgendo i clienti in una nuova dimensione.
Sfruttiamo le tecnologie IoT per connettere dispositivi e sistemi, migliorando l’efficienza operativa e l’utilizzo dei dati.
Offriamo programmi di formazione completi per migliorare le competenze della tua forza lavoro, aumentando la produttività e l’innovazione.
Forniamo consulenza strategica per fusioni e acquisizioni, garantendo un’efficiente integrazione e generazione di valore.
Creiamo strategie di marketing basate sui dati per aumentare la visibilità online, l’interazione con i clienti e i tassi di conversione.
Progettiamo interfacce utente intuitive e coinvolgenti, migliorando l’esperienza dell’utente e l’usabilità del prodotto.
Utilizziamo l’intelligenza artificiale generativa e simulativa per creare modelli e simulazioni realistiche, che aiutano nella pianificazione di scenari e nella formulazione di strategie.
Offriamo consulenza strategica per una trasformazione digitale di successo, consentendo alle imprese di adattarsi e prosperare nell’era digitale.
Ottimizziamo e semplifichiamo i processi operativi (OP), aumentando l’efficienza e riducendo i costi.
Sfruttiamo la scienza dei dati per estrarre informazioni significative da complessi set di dati, guidando decisioni aziendali informate.
Gestiamo il ciclo di vita dei prodotti, dall’ideazione al lancio, garantendo un ingresso sul mercato di successo e una sostenibilità nel tempo.
Sviluppiamo Token Non-Fungibili (NFT), consentendo la proprietà digitale e la creazione di valore nel mondo virtuale.
Creiamo chatbot intelligenti per automatizzare il servizio clienti, migliorando il coinvolgimento e la soddisfazione.
ICAREX non solo guida l'innovazione, ma la vive in prima linea. Siamo stati i primi a sviluppare competenze cutting-edge in ambiti come l'Intelligenza Artificiale Generativa, portando la nostra profonda conoscenza tecnologica direttamente al vostro servizio. Abbiamo il polso sul mercato grazie alle nostre venture dirette, che ci permettono di unire conoscenza tecnologica e industriale per fornire soluzioni su misura per le vostre esigenze. In ICAREX, i vostri obiettivi diventano i nostri.

Nutrizione e salute mentale personalizzate con un’app mobile basata sull’AI.

Scopri come Conversale, sfruttando l’IA e l’apprendimento automatico, ha trasformato significativamente il panorama dell’e-commerce.
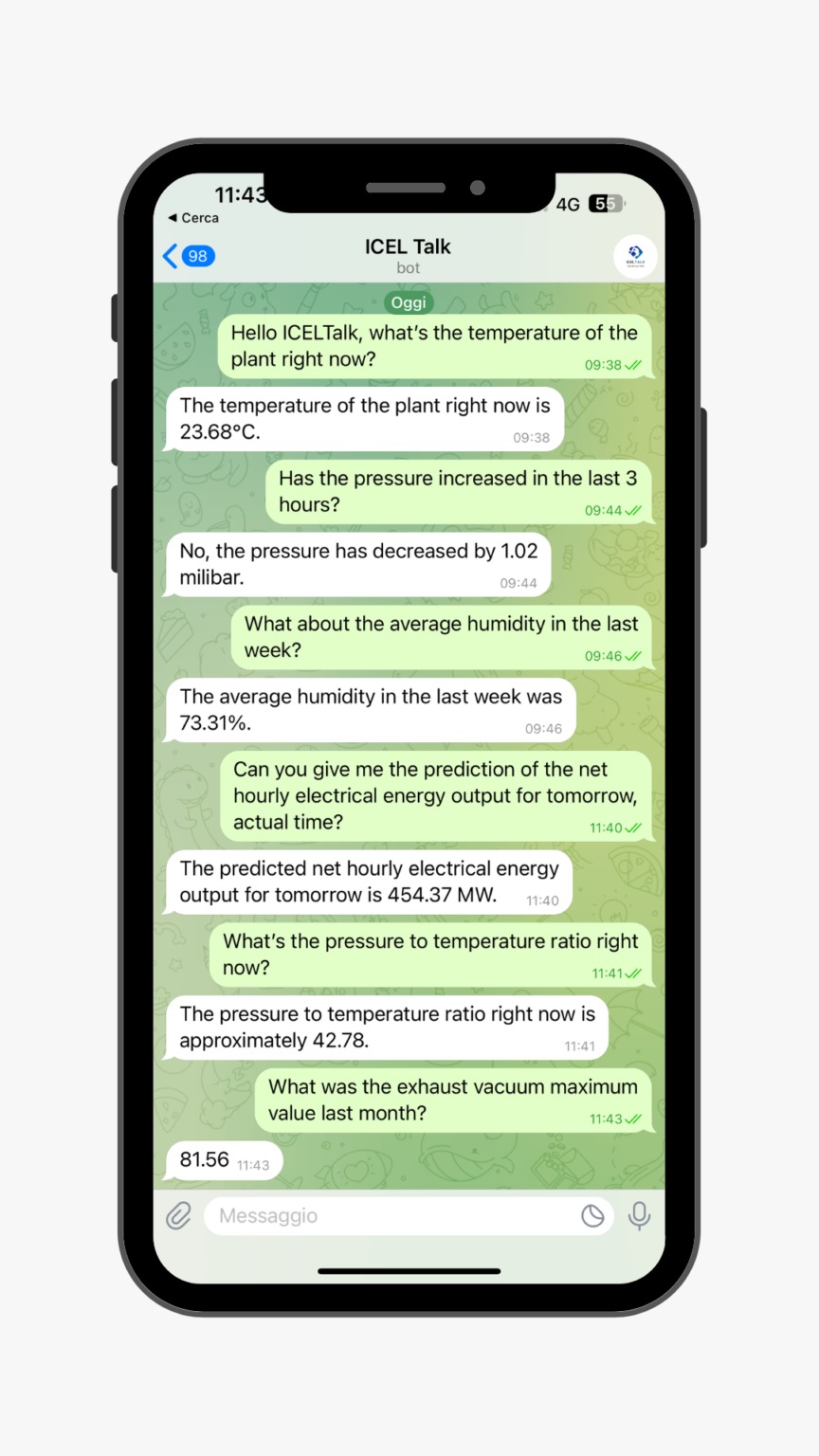
AI conversazionale progettata per aiutare i nostri clienti nel settore industriale a gestire e analizzare

Nel 2022 abbiamo acquisito ICEL, Dal 1935 leader nella Progettazione e sviluppo di Quadri ed Impianti Elettrici per l’industria ed il terziario. Questa mossa ha rafforzato la nostra capacità di fornire soluzioni di ingegneria elettromeccanica innovative e affidabili per i settori industriale e terziario, integrando le nostre offerte di software e AI.


Orgogliosi vincitori della competizione Startcup Lifescience, un testamento del nostro impegno per l’innovazione e l’eccellenza.
Il tuo percorso veloce verso l’innovazione.